Other stocks that surged for different reasons included Olipass, Noom, and Kainos Med.
Clinomics: Wild Price Swings Raise Concerns
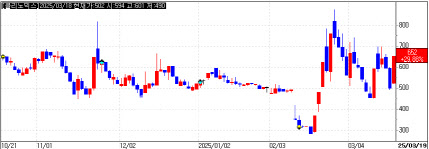
According to KG Zeroin MPDOCTOR, Clinomics closed at KRW 652, up 29.9% from the previous day, nearly doubling from its February low of KRW 283. The company specializes in liquid biopsy and diagnostic kits.
Clinomics’ stock has been highly volatile. It hit a high of KRW 878 on February 25 before plunging to KRW 480 over eight sessions. After briefly hitting the upper limit, it declined again. On March 18, the stock dropped 17%, only to surge back to the daily limit-up on March 19.
|
The recent rally appears driven by expectations of financial improvement and a company name change. Clinomics plans to rebrand as Celestra Inc. following a shareholder vote. The company also announced plans to issue KRW 5.5 billion (approx. $4.1 million) in convertible bonds, issuing 8.25 million new shares. Initially, the company aimed to raise KRW 10 billion ($7.5 million) but revised the issuance to KRW 5.5 billion to focus on a single investor, Dominate.
Structural Improvement Will Take Time; T&R Biofab Aims for KRW 30B Sales
Despite the rally, analysts warn that Clinomics still faces challenges, including the risk of delisting due to poor financials.
A company representative said, “We carefully selected investors based on their intent, financial capacity, and timing to ensure quick fundraising.”
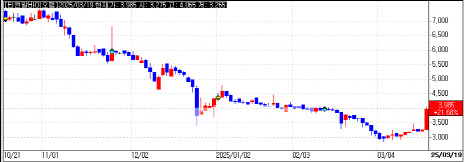
Meanwhile, T&R Biofab also saw its stock rise, closing at KRW 3,985, up 21.68%. The jump was linked to the company’s entry into the U.S. cosmetic market. T&R Biofab projects KRW 30 billion ($22.5 million) in revenue for 2024, a sixfold increase from KRW 4.9 billion ($3.7 million) in 2023.
|
However, T&R Biofab faces regulatory issues. The Ministry of Food and Drug Safety (MFDS) imposed a one-month, seven-day production suspension for violations of the Medical Device Act. The suspension runs from March 12 to April 18.
Authorities found pigskin residues in filtration systems and rust on mesh jigs in the company’s cleanroom. Regulators criticized T&R Biofab for failing to maintain hygienic production facilities, a requirement under medical device regulations.



!['과대망상'이 부른 비극…어린 두 아들 목 졸라 살해한 母[그해 오늘]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021700001t.jpg)
