◇‘Another Recognition of Technological Innovation’
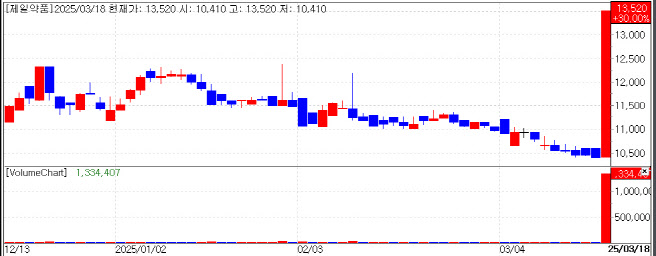
According to KG Zeroin’s MP Doctor (formerly MarketPoint), Jeil Pharmaceutical closed at KRW 13,520, up KRW 3,120 (30%) from the previous trading day. Its subsidiary, Onconic Therapeutics, ended at KRW 18,040, up KRW 4,160 (29.97%). The holding company, Jeil Pharma Holdings, also climbed 14.6% to KRW 8,400.
|
Nesuparib was previously granted orphan drug designation in 2021 for pancreatic cancer by both the FDA and South Korea’s Ministry of Food and Drug Safety (MFDS). This latest designation further underscores Onconic’s technological innovation and the potential of its cancer therapies.
Nesuparib inhibits two proteins, PARP (Poly ADP-ribose polymerase) and Tankyrase, both crucial for cancer cell survival, making it a next-generation targeted anticancer agent.
With the FDA’s orphan drug designation acceptance rate standing at just 17.6%, industry insiders see Nesuparib’s designation as significant both in terms of technical credibility and market potential. The high final approval rate for FDA-designated orphan drugs further supports expectations for Nesuparib’s commercialization. Notably, nearly 49% of new drugs approved by the FDA in 2022 had received orphan drug designation.
A Jeil Pharmaceutical representative commented on the stock’s rise: ‘If Nesuparib’s market value is fully recognized, it will drive corporate growth. By securing revenue through proprietary drugs and continuing new drug research, we anticipate a corporate value re-evaluation.’
Onconic is currently conducting a Phase 1b/2 clinical trial for pancreatic cancer and a Phase 2 investigator-initiated trial for endometrial cancer in combination with Keytruda. The latest orphan drug designation suggests that Onconic may soon enter clinical trials for gastric cancer as well. The company is scheduled to present its Nesuparib research findings at the American Association for Cancer Research (AACR) 2025 in April.
◇Boost to License-out and Investment Efforts
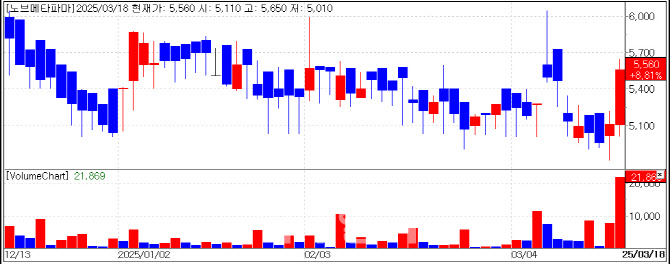
Novmeta Pharma’s stock surged 8.81% to close at KRW 5,560 on March 18. Given the 15% daily price limit in the KONEX market, this represents a significant gain. The rally was driven by a PharmEdaily pay-to-read article titled “PTC CEO Joins Novmeta Pharma with LOC in Hand for a $2 Trillion Blockbuster”, which was published on March 17 and made free on March 18 at 9:56 a.m.
|
기자 Pick
Peltz will oversee Novmeta Pharma’s global business operations. Joining him is Kylie O’Keefe, PTC’s former Chief Commercial Officer (CCO), who led global commercialization strategies for six rare neurological and metabolic disease products across 50+ countries. At Novmeta Pharma, she will serve as Chief Operating Officer (COO).
The two executives reportedly approached Novmeta Pharma on their own, recognizing the potential of its key pipeline. Their involvement helped the company secure a Letter of Cooperation (LOC) from a leading Canadian institution specializing in hereditary muscle disorders.
The company aims to focus on license-out deals and attracting investment, with plans to reattempt a KOSDAQ listing in the second half of the year.
◇Pioneering the First Osteoarthritis Disease-Modifying Drug
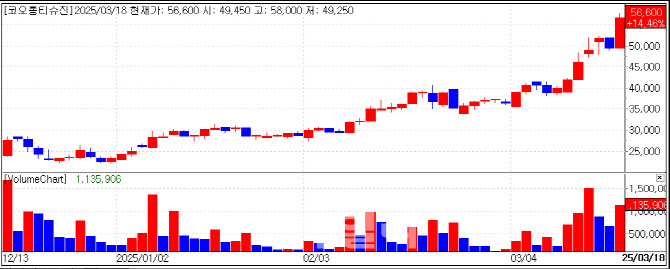
Kolon TissueGene jumped 14.46% to KRW 50,660 on growing anticipation for FDA approval of its osteoarthritis gene therapy, TG-C.
The stock has been on an upward trajectory, rising approximately 134% since the beginning of the year. Kolon TissueGene completed large-scale Phase 3 dosing for TG-C in 2024 and plans to file a Biologics License Application (BLA) with the FDA by 2026 following patient follow-ups.
|
Kolon TissueGene aims to obtain disease-modifying osteoarthritis drug (DMOAD) status from the FDA upon approval. Currently, no osteoarthritis drug has received DMOAD designation.
In a corporate report, Korea Investment & Securities analyst Hae-joo Wi stated: “If TG-C is approved as a disease-modifying osteoarthritis drug, sales could reach $7-8.2 billion, with an estimated operating profit of KRW 5.5 trillion.”
Commenting on the stock’s rise, a Kolon TissueGene spokesperson said: ‘Following our acquittal last November, market trust in the company has been restored. Recent U.S. media briefings have likely fueled optimism regarding TG-C’s FDA approval prospects.’
TG-C was originally launched in South Korea in 2017 but had its approval revoked in 2019 due to incorrect documentation on cell origins. The company has been involved in ongoing legal proceedings, though a 2023 criminal trial ruled in its favor.








![“우린 전생에 연인” 아빠가 친딸 건드리며 한 말 [그해 오늘]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/03/PS25032501644t.jpg)