◇ Onconic Therapeutics hits upper limit on cancer drug progress
Onconic Therapeutics, a drug development subsidiary of Jeil Pharmaceutical, closed at KRW 25,950 on the Kosdaq?up 29.9% from the previous day, hitting its daily upper limit. This marks two consecutive trading sessions of gains. The stock has more than doubled since March 11, when it hovered at KRW 12,250.
Established in 2020 to spearhead Jeil Pharmaceutical’s new drug R&D, Onconic went public on the Kosdaq in December 2023. The company attracted attention after its gastroesophageal reflux disease treatment, Zacubo, was approved as Korea’s 37th locally developed novel drug.
|
A company official said, “Nesuparib’s unique mechanism and innovative preclinical results were likely key factors in its review for FDA Orphan Drug Designation,” adding that the firm aims to expand indications through further Phase 2 trials.
Onconic is also drawing attention for its ambitions in Greater China. In March 2023, the company signed an exclusive licensing agreement with China’s Livzon Pharmaceutical Group, granting rights for development, regulatory approval, manufacturing, and commercialization of Zacubo in the region. Livzon is currently conducting a Phase 3 trial in China.
The deal is valued at up to $127.5 million (approximately KRW 165 billion), with Onconic already receiving a milestone payment of KRW 4.6 billion. Zacubo, a P-CAB (potassium-competitive acid blocker) drug, is the third of its kind to enter the market following HK Inno.N’s K-Cab and Daewoong’s Fexuclu. The company has also signed additional license-out agreements for 21 countries, including India, Mexico, and other Latin American markets.
A company representative commented, “We can’t speculate on share price movements, but as for our Greater China strategy, Zacubo is currently in Phase 3 trials in that region.”
◇ Mezzion gains on renewed hopes for Fontan drug
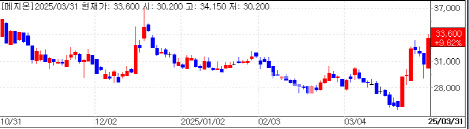
Shares of Mezzion also rose sharply. The company’s stock closed at KRW 33,600 in regular trading, up about 10%, and ended at KRW 34,100, up 11.3% on NXT, an alternative trading platform open until 8 p.m.
The gains are tied to expectations for U.S. and European approvals of JURVIGO, a treatment for Fontan patients. The drug is currently in a U.S. Phase 3 trial (FUEL-2) under the FDA. Mezzion had initially sought FDA approval in 2022 but was advised that its post-hoc analysis?excluding “super Fontan” patients?was not sufficient for regulatory review. The company has since redesigned its clinical trial for a renewed submission.
|
Plasmapp edges up despite financial concerns
Plasmapp shares also climbed 9% to close at KRW 725, despite being flagged as a possible management-risk stock. The company’s price has risen modestly from this month’s low of KRW 522 but remains significantly below its early-year high of KRW 1,485.
Plasmapp, a maker of plasma-based sterilizers and related consumables, has been grappling with financial strain. As of the first half of 2023, its cash and equivalents stood at just KRW 2.7 billion, and its capital was deeply in the red. Although Kospi-listed Dreamtech injected KRW 15.3 billion in a rights offering last July, the company’s cash balance had dwindled to KRW 1.5 billion by Q3’s end.
A company official said, “To improve our financial structure and offset accumulated losses, we’ve decided to reduce capital by 90%,” adding that the record date is April 14, 2025, with new shares expected to be listed around May 9.
Plasmapp reported KRW 10.1 billion in revenue in 2023 and an operating loss of KRW 17.6 billion.