|
◇Alteogen Declines on Speculation Over Keytruda SC Phase 3 Trial Results
Alteogen’s stock suffered from market speculation about the upcoming Phase 3 results of Keytruda SC (subcutaneous). Rumors suggested that if the SC formulation fails to show improved efficacy over the IV (intravenous) version, the stock could drop further.
The European Lung Cancer Congress (ELCC) abstract on Keytruda SC‘s Phase 3 results is set for release on March 27. Merck previously stated that Keytruda SC demonstrated efficacy equivalent to the IV formulation.
Eom Min-yong, an analyst at Shinhan Investment Corp., refuted the negative speculation, saying, “Unfounded comments are circulating on social media, suggesting that Keytruda SC will fail to meet expectations if it does not outperform the IV version. This misinformation needs to be corrected.” He added, “These comments appear to be misleading and come from those who do not understand Merck’s Keytruda SC trial design.”
Eom emphasized that Keytruda currently generates about $30 billion in annual revenue from its IV formulation alone, with an additional $3 billion to $4 billion in yearly growth. He added that transitioning to SC would improve patient convenience and reduce hospitalization costs, accelerating market adoption.
He also pointed out that Roche’s Tecentriq SC, despite taking 7~8 minutes for administration (compared with IV’s 30~60 minutes), captured 32% of the IV market within nine months. In contrast, Keytruda SC takes just two to three minutes, while its IV formulation requires four to five hours.
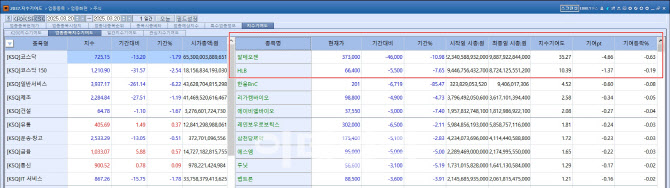
◇HLB Stocks Drop Ahead of FDA Decision
HLB Group stocks continued their downturn, with HLB closing at 66,400 won, down 7.65% (5,500 won). Other HLB affiliates also posted losses: HLB Biostep (-6.21%), HLB Pharma (-5.61%), HLB Global (-5.48%), HLB Life Sciences (-5%), HLB Therapeutics (-4.6%).
The decline was attributed to growing concerns over HLB’s liver cancer drug, Rivoceranib, and its pending U.S. Food and Drug Administration approval. HLB Group announced that it would disclose the FDA’s new drug application decision via YouTube on March 21.
Market sentiment turned cautious as the FDA typically announces approvals quickly when decisions are positive. For instance, Yuhan Corp.’s lung cancer drug, LECLAZA, received FDA approval two days before its review deadline. The fact that Rivoceranib’s review period is being fully utilized has fueled uncertainty in the market.
An HLB spokesperson said, “As soon as we receive the decision, we will announce it on our website and YouTube channel.”
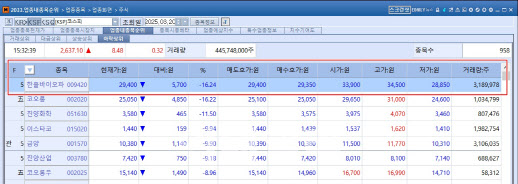
◇HanAll Biopharma Plunges Despite Phase 3 Success for ‘Batoclimab’
In the KOSPI market, HanAll Biopharma’s sharp decline stood out, as the stock fell 16.24% despite announcing positive Phase 3 results for its antibody drug Batoclimab.
|
The primary endpoint measured improvement in daily living activities (MG-ADL score) at week 12. The results showed: Batoclimab 680 mg group: 5.6-point improvement (p<0.001), Batoclimab 340 mg group: 4.7-point improvement (p<0.05), Placebo group: 3.6-point improvement.
The company plans to analyze the data before submitting a regulatory application in Japan for autoimmune disease treatment.
However, the sharp stock decline was largely due to the ongoing arbitration dispute between HanAll Biopharma and China’s Harbour BioMed over Batoclimab’s licensing rights.
On March 18, HanAll Biopharma and Harbour BioMed entered arbitration proceedings regarding their 2017 licensing agreement for Batoclimab.
Earlier this year, HanAll Biopharma notified Harbour BioMed of contract termination and rights reversion, citing Harbour BioMed’s failure to meet its contractual obligations. In response, Harbour BioMed filed for arbitration with the International Chamber of Commerce, asserting that the agreement remains valid.
A HanAll Biopharma spokesperson clarified, “The arbitration process is unrelated to Batoclimab’s efficacy and safety. The licensing agreement with Harbour BioMed remains valid until arbitration concludes, and the regulatory filing for gMG remains on track.”
The company added, “We will actively challenge Harbour BioMed’s claims through legal counsel and review whether the licensing agreement has been properly upheld.”








![“우린 전생에 연인” 아빠가 친딸 건드리며 한 말 [그해 오늘]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/03/PS25032501644t.jpg)